General Information
The main host of the lethal yellowing (LY) disease is Cocos nucifera. At least 30 other species of palms are susceptible to lethal yellowing and/or lethal yellowing-type disease. LY disease on African oil palm (pure E. guineensis stands, nonhybrids) has been reported from Colombia in 1963. The disease incidence on commercial oil palms today has been significantly reduced, as the planting materials are of E. guineensis x E. oleifera hybrid – E. oleifera has resistance to this fatal disease.
The causal factor is the phytoplasmas, tiny cell wall-less prokaryotes (142-295 nm in diameter and may vary from 1 to 16 μm in length) that can only be visualised using transmission electron microscopy. In diseased palms, the LY phytoplasmas are normally found within the phloem sieve tubes.
LY involves a prolonged a symptomless phase. During this incubation period, the phytoplasma will induce several metabolic changes – stimulation of plant growth, which is followed by a phase gradual decline before a complete stop of vertical growth. External symptoms begin to show only after 112 to 262 days from infection.
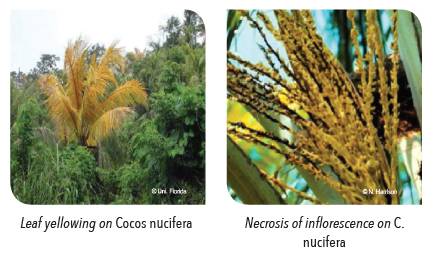
Early symptoms include: i) premature shedding of all fruit (nutfall); aborted nuts develop necrotic brown-black calyx end rot; and ii) inflorescence necrosis (occurs after nutfall). As the disease progresses, the additional emergent inflorescences show more extensive necrosis (usually at the tips of flower spikelets) and may be totally discolored. Subsequently, all male flowers die and the palm would not be able to set fruits.
Yellowing of the leaves begins once two or more inflorescences develop necrosis. Leaf discolouration occurs rapidly, starting with the older and lowermost leaves, and spreads upward to the crown. Yellowed leaves turn brown, desiccate and die. In some cases, leaf yellowing occurs on a single yellow leaf (flag leaf) in the mid-crown. Affected fronds often hang downwards for several days before falling off. A putrid basal soft rot of the newly emerged spear (youngest leaf) occurs during the advance stage of leaf yellowing. Spear leaf dies, the tip of the apical meristem rots and the entire crown foliage falls off leaving a bare trunk. Infected palms usually die within 3 to 6 months after the appearance of the first symptoms.
Phytoplasmas are transmitted by phloem – feeding insect vectors (or sap feeders) during feeding. The vector of Ca. Phytoplasma palmae is the planthopper Haplaxius (= Myndus) crudus (American palm cixiid). M. crudus occurs in the North, Central and South America, and in the Caribbean region. Other sap feeders (e.g. Haplaxius sp. and Cedusa sp.) can potentially vectors LY phytoplasmas. Phytoplasmas can also spread through infected propagative plant material.
Distribution
Africa, Latin America.
Detection and Inspection
No single symptom is diagnostic of LY. Symptoms may vary depending on palm species/cultivar. It is the progression of the series of symptoms that distinguishes LY from other diseases with similar symptoms. Visual examination for LY symptoms during initial infection stage is not possible due to the symptomless incubation period. To date, no biological or serological tests for detection of the LY phytoplasma have been successfully developed as phytoplasmas are obligate parasites. PCR is the most sensitive test currently available for phytoplasma detection. The methodology involves the amplification of the 16S rRNA genes, digestion of the amplicons using AluI, HinfI, TaqI or Tru9I endonucleases, and running the fragments through polyacrylamide gel electrophoresis (PAGE) for identification of the LY group 16SrIV phytoplasmas. The differentiation of the 16SrIV-A subgroup responsible for LY in palms can be made by using the non-ribosomal primer pair LYF1/LYR1.

Prevention and Control
PHYTOSANITARY
The LY phytoplasmas is listed as an A1 quarantine pest by EPPO, and quarantine pest of high concern by APPPC, CPPC, IAPSC and NAPPO.
The import of germplasm material (seeds, pollen, tissue culture) must be accompanied by an import permit issued by or on behalf of the Director-General Department of Agriculture for Peninsular Malaysia (including Labuan), or the Director Department of Agriculture for Sabah, and a phytosanitary certificate issued by an authorised official from the country of export. The import conditions are available upon request from the Plant Biosecurity Division Malaysia. All consignments are subjected to inspection by the Department of Agriculture prior to clearance by Customs. Germplasm material imported from high risk areas should be sent for third country quarantine before arrival onto Malaysian shores. The import of alternative host plants e.g. Phoenix dactylifera and other ornamental palms from infested areas should be enquired with DOA.
CULTURAL CONTROL AND SANITARY METHODS
Best control is to plant resistant varieties. Insecticidal control of vectors/potential vectors (i.e. planthoppers) can limit the spread of the disease.
Treatment of infected palms by injection into the trunk of oxytetracycline-HCl has been shown to control the symptoms of lethal yellowing. Retreatment to be applied at 4-monthly intervals.
Further reading
- ABI Invasives Species Compendium (2014). Candidatus Phytoplasma palmae (lethal yellowing of coconut). Url: http://www.cabi.org/isc/datasheet/38647.
- CABI and EPPO (2014). Palm lethal yellowing phytoplasma. URl: http://www.eppo.int/QUARANTINE/ data_sheets/ bacteria/PHYP56_ds.pdf.
- Sullivan, M and Harrison, N (2013). CPHST Pest Datasheet for ‘Candidatus Phytoplasma palmae’ and related strains. USDA-APHIS-PPQ-CPHST. Revised June, 2014.
- Smith, N (2015). Palms and People in the Amazon. Springer International Publishing: Switzerland. 231 pp.

