General Information
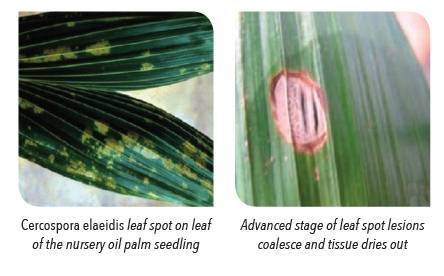
Disease of nursery seedlings, which sometimes start in prenursery and is frequently carried to field plantings, where it can survive for many years.
The youngest leaves of nursery seedlings become infected and small translucent spots surrounded by yellowish- green haloes enlarge and become dark brown (≤ 0.5 mm in diameter). Conidiophores emerging through the stomata in the centre of the spots, mainly on the underside of the leaf, produce conidia which give rise to further, surrounding spots. This results in a freckled appearance, but later the lesions coalesce (fully developed spots are 3-4 mm in diameter and the tissue dries out to become greyish-brown and brittle. Heavily infected tissues die. The disease tends to become aggressive as the leave age, and the process may proceed very rapidly during wet seasons. In West Africa, this is usually the middle or end of the wet season, and in the following dry season, the dying out of the older leaves is compounded by Cercospora infection.
C. elaeidis can reduce yields by more than 10% over the first 7 years of production. Even moderate attacks can reduce the green leaf area and might therefore affect early bunch production.
Distribution
Africa (Central, East and West regions); Australia (Northern Territory – recorded on Carpentaria acuminata); Suriname; Thailand.
Detection and Inspection
During initial stages of infection, small translucent spots surrounded by yellowish-green haloes form and enlarge into dark brown spots (≤ 0.5 mm in diameter). Infected leaves develop freckled appearance. The lesions coalesce to form brown spots (3-4 mm in diameter) with an outer orange ring. The infected area dries out to into greyish brown and becomes brittle.
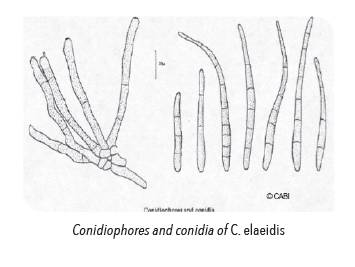
Detailed examination of sample in the laboratory is needed to confirm the identity of the fungal pathogen. The conidiophores in fascides of 2-7, divergent, erect, geniculate, base swollen, dark brown, rough or finely echinulate, septate, conidial scars conspicuous, 185-250 x 5-7 μm. Conidia obclavate, base truncate, apex tapered, yellowish-brown, with the apical region paler or hyaline, 0-9-septate, finely echinulate, hilum thickened, 125- 187 x 6-10 μm.
Prevention and Control
PHYTOSANITARY
Fungicides e.g. benomyl + thiram / Dithane 45 can be used to control disease incidence and wash seeds. The import of germplasm material (seeds, pollen, tissue culture) must be accompanied by an import permit issued by or on behalf of the Director-General Department of Agriculture for Peninsular Malaysia (including Labuan), or the Director Department of Agriculture for Sabah, and a phytosanitary certificate issued by an authorised official from the country of export. The import conditions are available upon request from the Plant Biosecurity Division Malaysia. All consignments are subjected to inspection by the Department of Agriculture prior to clearance by Customs. Germplasm material imported from high risk areas should be sent for third country quarantine before arrival onto Malaysian shores.
CULTURAL CONTROL AND SANITARY METHODS
The spread of C. elaeidis should be controlled within nurseries. Control of freckle with fungicides (e.g. benomyl) is more effective if done at nurseries. Alternatively, all old dry leaves and other badly infected leaves of oil palms in nurseries should be removed by pruning and destroyed. Fungicide control in the field is has not been as effective. No other method has been proven effective. Wider spacing of seedlings and techniques that involve the minimum of transplanting shock may reduce the level of field infection.
Further reading
- Cab International (1998). Cercospora elaeidis. CMI Descriptions of Pathogenic Fungi and Bacteria No. 464. Set No. 47, published 1975.
- Corley, R H V and Tinker, P B (2003). The Oil Palm 4th ed. Blackwell Science Ltd: UK. 592 pp.
- Pornsuriya, C; Sunpapao, A; Srihanant, N; Worapattamasri, K; Kittimorakul, J; Phithakkit, S and Petcharat, V (2013). A survey of diseases and disorders in oil palms of Southern Thailand. Plant Pathology J. 12(4): 169-175.
- Holliday, P (1980). Fungus diseases of tropical crops. Cambridge University Press: UK. 69 pp.
- Singh, B P (ed.) (2013). Biofuel Crops: Production, Physiology and Genetics. CABI: UK. 397 pp.

