General Information
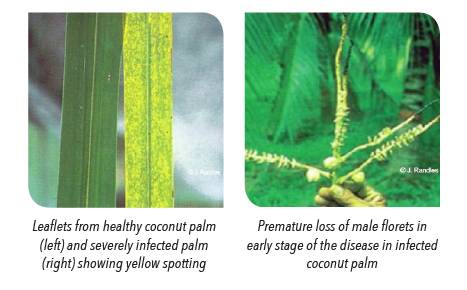
Coconut cadang-cadang viroid (CCCVd) is the smallest known viroid. Its size varies from 246 to 247 nucleotides during early stage of infection stage to 296 to 297 nucleotides during later stage of infection stage.
The name cadang-cadang is derived from ‘gadan-gadan’, which means dead or dying. It is mostly used to refer to premature decline and death of coconut palms in the Philippines. Annually, approx. 500 000 coconut palms die from CCCVd in the Philippines. The disease can be naturally spread or inoculated onto other palm species including oil palm and herbaceous monocotyledons in the Phillipines. It has also been observed that oil palm is more susceptible to CCCVd compared to coconuts.
CCCVd occurs in the vascular tissue and mesophyll cells of a host. The viroid is present in the husk, the embryo of nuts and pollen. It has been postulated that the viroid can be transmitted by insects through feeding wounds, but no vector has been identified so far. CCCVd can spread through mechanical inoculation e.g. contaminated machetes and other tools.
Distribution
CCCVd: Philippines, Solomon Islands.
Detection and Inspection
CCCVD
Symptoms are unreliable in the detection of CCCVd as the infection can remain latent. The presence of viroid can only be detected and identified through molecular diagnostic tools.
CCCVd can be detected using 1- or 2-dimensional polyacrylamide gel electrophoresis (PAGE). Optimum resolution of CCCVd and its sequence variants can be obtained by increasing the concentration of polyacrylamide from the standard 5% to 20% under non-denaturing conditions. The nucleic acid bands are visualised by staining with silver nitrate. The PAGE assay is suitable for detection of CCCVd in leaf samples where the concentration of CCCVd is usually above 50 ng and to determine stage of infection. This technique is preferred for field surveys and disease monitoring.
Dot-blot molecular hybridisation is a more sensitive method to detect viroid presence before symptoms develop. CCCVd is cloned or amplified by the polymerase chain reaction (PCR) and the clones or PCR product are used as templates for synthesis of radioactively-belled complementary nucleic acid probes. Extracts of leaf samples can la be probed on nitrocellulose sheets with 325 labeled DNA probes. The presence of the viroid is detected by its specific hybridisation with the probe. The radioactive label causes darkening of exposed x-ray film, while samples without the viroid will show no signal. Another method approved for CCCVd detection is a one-tube reverse-transcriptase polymerase chain reaction (RT- PCR). Three sets of primers are used: primer G2-1 for RT-PCR and primers G2-1 and D9-1 for conventional PCR. The priming sites are located at the central conserved and pathogenicity regions of CCCVd. PCR products are then visualised using gel electrophoresis and ethidium bromide staining.
Prevention and Control
PHYTOSANITARY
CCCVd viroids can be transmitted by seed or pollen and occur in almost all plant parts. The import of germplasm material (seeds, pollen, tissue culture) must be accompanied by an import permit issued by or on behalf of the Director-General Department of Agriculture for Peninsular Malaysia (including Labuan), or the Director Department of Agriculture for Sabah, and a phytosanitary certificate issued by an authorised official from the country of export. The import conditions are available upon request from the Plant Biosecurity Division Malaysia. All consignments are subjected to inspection by the Agricultural Department prior to clearance by Customs. Germplasm material imported from high risk areas should be sent for third country quarantine and molecular diagnosis before arrival onto Malaysian shores.
The import of primary and alternative palm hosts i.e. Cocos nucifera and Corypha elata from infested areas should be enquired with DOA.
CULTURAL CONTROL AND SANITARY METHODS
No known control measures exist for CCCVd in the field.
Further reading
- CABI Invasive Species Compendium (2014). Datasheet report for coconut cadang-cadang viroid (Cadang-cadang disease). URL: http://www.cabi.org/isc/ datasheet/13700.
- Eppo and CABI (undated). Coconut Cadang-cadang viroid. URL: http://www.eppo.int/QUARANTINE/data_ sheets/virus/ CCCVD0_ds.pdf.
- Hanold, D and Randles, J W (1991). Coconut Cadang-cadang disease and its viroid agent. Plant Dis. 75(4): 330-335.
- Sullivan, M; Daniells, E and Robinson, A (2012). CPHST Pest Datasheet for Coconut Cadang-cadang Viroid. USDAAPHIS- PPQ-CPHST.
- Turner, P D and Gillbanks, A (2003). Oil palm cultivation and management. 2nd ed. The Incorporated Society of Planters: Malaysia. p. 649-651.
- Wu, Y H; Cheong, L C; Meon, S; Lau, W H; Kong, L L; Joseph, H and Vadamalai, G (2012). Characterization of Coconut Cadang-cadang Viroid variants from oil palm affected by orange spotting disease in Malaysia. Arch. Virol. 158(6): 1407-1410.
- Rodriguez, M J B (2003). Coconut and other palm trees: Viroid diseases. In: Leobenstein, G and Thottappilly, G (eds.) Virus and virus-like diseases of major crops in developing countries. Vol. 1. Springer Science+Business Media BV: Netherlands. p. 573-574.

